Ozempic (semaglutide) has transformed weight management, but emerging research has revealed concerning links between this popular GLP-1 receptor agonist and serious eye complications. The most alarming finding involves non-arteritic anterior ischemic optic neuropathy (NAION).
A study showed that diabetic patients taking semaglutide were over four times more likely to be diagnosed with NAION than those on other medications. For patients using Ozempic for weight loss, the risk is even more pronounced. Those prescribed the drug for obesity were more than seven times more likely to receive a NAION diagnosis than similar patients who didn’t take semaglutide.
Beyond NAION, Ozempic can cause temporary blurred vision and may accelerate diabetic retinopathy progression, particularly during the first few months of treatment. Understanding these risks is essential for anyone considering or currently taking semaglutide medications.
TL;DR
- Ozempic (semaglutide) has been linked to an increased risk of non-arteritic anterior ischemic optic neuropathy (NAION), a rare but serious eye condition that can cause sudden, permanent blindness.
- Patients with diabetes taking semaglutide are about four times more likely to develop NAION, while those with obesity are about seven times more likely to experience this condition compared to non-users.
- The European Medicines Agency classifies NAION as affecting up to 1 in 10,000 users.
- While the absolute risk remains low, anyone taking Ozempic should have baseline eye exams and report sudden vision changes immediately.
What Are Ozempic Eyes? NAION Explained
“Ozempic eyes” refers to a constellation of vision-related side effects associated with semaglutide use, most notably NAION (non-arteritic anterior ischemic optic neuropathy). NAION occurs when blood flow to the optic nerve stops, and researchers believe GLP-1 drugs could affect blood flow to the optic nerve, leading to this devastating condition.
NAION presents as sudden, painless vision loss, typically affecting one eye without warning. More than 1 million nerve fibers within the optic nerve relay visual signals from the eyes to the visual parts of the brain, and damaged fibers can disrupt the crucial eye-brain connection, leading to blindness.
According to ophthalmology experts, NAION is the most common cause of sudden or severe optic nerve injury in people older than 50.
How Ozempic Causes NAION?
The mechanism behind semaglutide-induced NAION remains under investigation. Most neuro-ophthalmologists attribute NAION to reduced blood pressure, as when blood pressure drops too much, it can reduce the delivery of oxygen and other nutrients below a critical threshold that causes injury to the nerve fibers. Since semaglutide can lower blood pressure, this may explain the connection.
Ozempic Eye Conditions
| Condition | Risk Level | Temporary? | Primary Symptoms |
| NAION | 4-7x increased risk | No (permanent) | Sudden one-eye blindness, painless |
| Blurred Vision | Common (10-20%) | Yes | Vision fluctuations from blood sugar changes |
| Diabetic Retinopathy | Increased early (months 1-6) | Often reversible | Dark spots, floaters, bleeding |
| Eye Floaters | Not directly related | N/A | Normal vitreous aging (age 50+) |
It’s important to distinguish NAION from common age-related floaters. Eye floaters result from vitreous changes that naturally occur with aging, particularly after age 50, and ophthalmologists emphasize that these are typically unrelated to Ozempic use itself.
Ozempic NAION Studies – Risk Analysis
A landmark research establishes the Ozempic-NAION connection, in which among 710 patients with type 2 diabetes, there were 17 cases of NAION in patients prescribed semaglutide, translating to a cumulative rate of 8.9% over three years, compared with six cases in patients prescribed non-GLP-1 diabetes drugs at a rate of 1.8%.
Through statistical analyses, researchers estimated there was a 4.28 times greater risk of developing the condition in patients prescribed semaglutide. For the obesity cohort, the findings were even more striking, with patients showing more than sevenfold increased risk.
International studies have corroborated these findings. A study determined that using once-weekly semaglutide doubles the five-year risk of developing NAION in those who have type 2 diabetes.
A 2025 study presented at the American Academy of Ophthalmology found that people using GLP-1 drugs were 68.6 times more likely to develop NAION and eight times more likely to develop diabetic retinopathy than those taking alternative diabetes medications.
Non-Ozempic Diabetes NAION Connection
It’s crucial to understand that people with diabetes and obesity already face elevated NAION risk regardless of medication. Diabetes itself approximately doubles baseline NAION risk, making the interaction between disease state and medication particularly important.
Regarding floaters specifically, ophthalmologists have consistently emphasized these are not directly Ozempic-related but rather result from normal vitreous detachment that occurs with age, particularly in people over 50.
Does Blurred Vision from Ozempic Go Away?
Research confirms that approximately 90% of temporary blurring cases resolve within weeks 1-4 as blood sugar levels stabilize. Older patients are more likely to experience blurred vision when beginning the medication because the eye’s lens becomes less flexible as we age, so vision takes longer to stabilize when the body experiences these changes in blood sugar levels.
The “Ozempic eyes” blurring typically peaks during the initial titration period and steadily improves by month 2 and beyond. This transient effect differs fundamentally from permanent conditions like NAION.
However, persistent blurring lasting more than 2 months warrants immediate ophthalmological evaluation. While most vision changes are temporary and benign, sustained symptoms could indicate more serious complications like worsening diabetic retinopathy or, rarely, developing NAION.
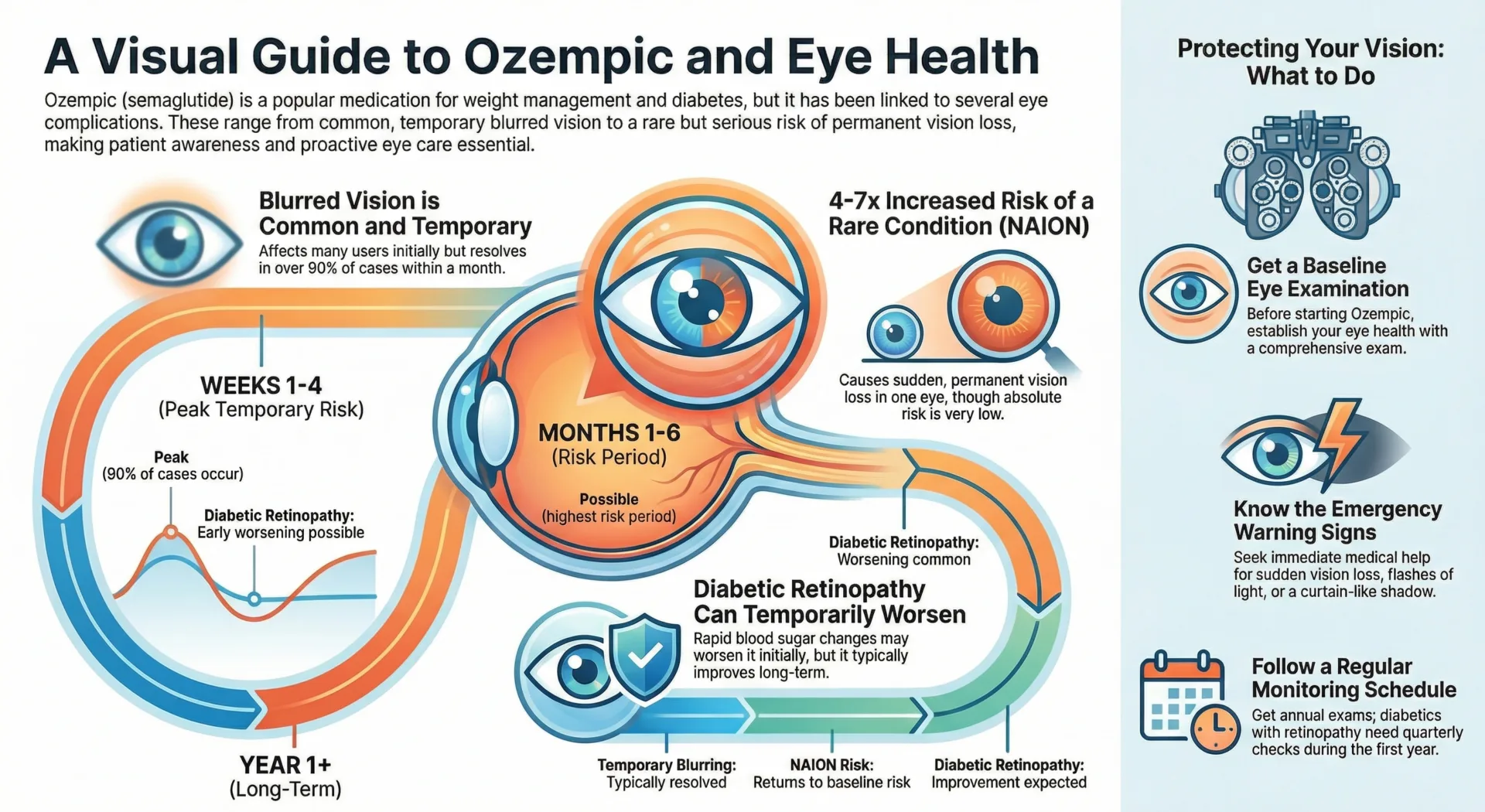
Ozempic Vision Risk Timeline
Understanding when different vision complications may occur helps patients and healthcare providers monitor appropriately:
Complete Ozempic Vision Risk Timeline
| Timeframe | Temporary Blurring Risk | Permanent NAION Risk | Diabetic Retinopathy Risk |
| Week 1-4 | Peak (90% of cases occur) | Low | Early worsening possible |
| Month 1-6 | Declining steadily | Possible (highest risk period) | Transient worsening common |
| Year 1+ | Typically resolved | Returns to baseline diabetes risk | Improvement expected with sustained control |
The first 4-6 months represent the critical monitoring period. Studies show this early worsening happens in the first year or two, but then provides much better control of retinopathy long term.
Most NAION cases reported in clinical studies occurred within the first year of semaglutide use, suggesting a drug-induced effect related to treatment initiation or rapid metabolic changes. However, the absolute risk remains very low – the European Medicines Agency estimates fewer than 1 in 10,000 patients will develop NAION.
Diabetic Retinopathy & Ozempic Eye Problems
Diabetic retinopathy (DR) presents unique challenges for Ozempic users. The paradox: while better long-term blood sugar control prevents diabetic eye disease, rapid glucose improvements can temporarily accelerate existing retinopathy.
With improved blood sugar and A1C, diabetic retinopathy and macular edema typically improve over time, but with Ozempic use about 10-20% of patients experience worsening retinopathy and macular edema. This percentage increases among patients with advanced retinopathy at baseline.
The mechanism involves vascular stress from rapid metabolic changes. Research shows that very rapid improvements in blood sugar control from medications like Ozempic can sometimes cause existing diabetic retinopathy to temporarily worsen, even though better blood sugar control is generally good for long-term eye health.
This early worsening manifests as:
- Increased vitreous hemorrhage (bleeding into the eye)
- New or worsening macular edema (retinal swelling)
- Progression of proliferative changes
- Increased floaters from vitreous shifts
Importantly, this appears to be a change early on (about three to six months) while taking the medication and should eventually improve the retinopathy and edema. The vast majority of patients who experience early worsening see stabilization and improvement with continued treatment.
Annual dilated eye exams remain essential for all Ozempic users, with diabetic patients requiring quarterly monitoring during the first year of treatment if they have existing retinopathy.
Ozempic Eye Safety Guidelines
Protecting your vision while using Ozempic requires proactive monitoring and clear communication with healthcare providers:
Baseline Eye Examination
Before starting Ozempic, a comprehensive ophthalmological evaluation is essential. This exam should include dilated fundoscopy, optic nerve assessment, and documentation of any pre-existing diabetic retinopathy or risk factors for NAION.
Emergency Warning Signs
Seek immediate medical attention for:
- Sudden vision loss in one eye (painless or otherwise)
- Persistent new floaters lasting more than 2 weeks
- Flashes of light or curtain-like vision loss
- New dark spots or “curtain” across vision
- Sudden changes in color vision
- Any abrupt, unexplained vision changes
Ongoing Monitoring Schedule
- Non-diabetic patients: Annual comprehensive eye exams
- Diabetic patients without retinopathy: Annual dilated exams
- Diabetic patients with existing retinopathy: Quarterly exams during year 1, then as recommended
Healthcare providers should also assess optic nerve anatomy. Patients with “crowded” optic discs face elevated NAION risk and require particularly careful monitoring.
NAION Risk Factors Beyond Ozempic
While Ozempic increases NAION risk, several pre-existing conditions contribute independently.
Primary Risk Factors:
- Age 50+ (most common demographic for NAION)
- Sleep apnea (often exceeds Ozempic risk contribution)
- Hypertension and cardiovascular disease
- Diabetes mellitus (doubles baseline risk)
- Small or “crowded” optic disc anatomy
- Previous NAION in the opposite eye
Sleep apnea deserves special attention. Its contribution to NAION risk may actually exceed that of semaglutide in many patients. High blood pressure combined with the blood pressure-lowering effects of Ozempic creates a compounded risk. Patients with multiple risk factors require especially vigilant monitoring.
References & Resources
Disclaimer: This information is intended for general knowledge and informational purposes only and does not constitute medical advice. Always consult with a healthcare professional for personalized guidance.
Written by the Pandameds.com Editorial Team
Our content is created by pharmacy-trained researchers and healthcare specialists and rigorously reviewed by a diverse panel of authentic experts from the pharmaceutical and healthcare fields. This collaborative review process ensures that every article meets the highest standards of medical accuracy, reliability, and relevance.
- ✅ Authored by pharmacy-trained professionals
- 🔍 Reviewed by multiple verified experts in the pharmaceutical and healthcare niche
- 💊 Based on trusted sources including FDA, Health Canada, and peer-reviewed clinical studies
- 🔄 Regularly reviewed and updated every 90 days to maintain accuracy and trustworthiness
About Pandameds.com
Pandameds.com offers a range of weight loss medications at an affordable price.
Fast, Reliable Shipping to the USA!
Affordable Prescription Meds From Canada
Join our mailing list for exclusive promos, curated health content & more.
Frequently Asked Questions
What is Ozempic vision loss?
Vision loss from Ozempic primarily involves NAION, a rare optic nerve stroke. Diabetes already doubles baseline NAION risk even without medication, making disease management complex.
Does Ozempic cause blindness?
NAION linked to Ozempic represents a 4-7x relative risk increase, but the absolute risk affects up to 1 in 10,000 people taking semaglutide. While serious, true blindness remains rare.
Does blurred vision from Ozempic go away?
Yes, in approximately 90% of cases, blurred vision resolves by month 2 as blood sugar levels stabilize and the eye's lens adapts to new glucose concentrations.
Does Ozempic cause eye problems?
Temporary blurring is common and resolves. NAION represents a rare but permanent risk requiring immediate medical attention if sudden vision loss occurs.
Are Ozempic and eye floaters related?
No direct relationship exists. Floaters result from normal vitreous aging changes, particularly common after age 50, and are not caused by semaglutide medication.
What are Ozempic eye symptoms?
Temporary blurring during weeks 1-4 is common. Sudden one-eye vision loss requires emergency room evaluation for possible NAION.
What is the Ozempic retinopathy risk?
Risk increases during months 1-6 from rapid A1C reduction, affecting 10-20% of diabetic patients with existing retinopathy. This typically reverses with continued treatment.
Are Ozempic vision changes permanent?
Approximately 90% of vision changes are temporary, resolving within 2 months. Permanent vision loss from NAION affects fewer than 0.01% of users.
How often should I have eye exams on Ozempic?
Baseline examination before starting, then annual exams minimum. Diabetic patients with retinopathy require quarterly monitoring during year 1.
Is it safe to continue Ozempic with blurred vision?
Temporary blurring is generally safe to monitor while continuing medication. However, watch carefully for NAION warning signs like sudden, painless vision loss requiring immediate discontinuation and medical evaluation.
Related Blog Posts
Call Us Today!
If you have any questions, please email our support team at support@pandameds.com or call us toll-free at 1-888-862-1210.