⚠️ Medical Disclaimer: This information is for educational purposes only and is not medical advice. Always consult your healthcare provider before starting or stopping any medication.
GLP 1 receptor agonists have transformed how we approach type 2 diabetes and obesity management. These medications, once primarily diabetes drugs to manage blood glucose levels, now stand as backbone therapies for complex cardiometabolic conditions. The rapid expansion of this drug class since 2005 reflects growing recognition that managing blood sugar and body weight requires sophisticated, multi system approaches rather than single organ treatments.
GLP-1 Receptor Agonists: What They Are and How They Work
GLP 1 receptor agonists activate specialized receptors throughout your body to improve glucose control, reduce appetite and support weight management. Recent expert consensus from 2023–2025 characterizes these medications as metabolic disease modifiers rather than simple glucose lowering agents. They work through integrated actions on your pancreas, gut-brain axis and multiple organs including the liver, heart, kidney and brain.
Quick Fact Table: GLP-1 Agonists List & Key Features
| Medication | Active Ingredient | Dosing | Primary Use | Weight Loss |
| Ozempic | Semaglutide | Weekly | T2DM, CV risk reduction | High |
| Wegovy | Semaglutide | Weekly | Obesity/weight management | Highest |
| Trulicity | Dulaglutide | Weekly | T2DM, heart benefits | Moderate |
| Victoza | Liraglutide | Daily | T2DM | Moderate |
| Mounjaro | Tirzepatide (GLP-1/GIP) | Weekly | T2DM, obesity | Highest |
| Rybelsus | Semaglutide (oral) | Daily | T2DM | Moderate |
What Are GLP-1 Receptor Agonists?
GLP 1 receptor agonists are injectable or oral medications that mimic your body’s natural glucagon like peptide 1 hormone. These drugs bind to GLP 1 receptors on pancreatic, gastrointestinal and central nervous system cells to enhance glucose dependent insulin secretion. This glucose dependent mechanism means they lower blood sugar primarily when it’s elevated reducing hypoglycemia risk compared with older diabetes medications.
Contemporary reviews describe GLP 1 medications as incretin based therapies that produce clinically meaningful HbA1c reductions in trials along with substantial, dose dependent weight loss when prescribed at obesity indicated doses. The drug class now includes multiple formulations from twice daily injections to once weekly options and even oral tablets providing flexibility for different patient needs and preferences.
GLP-1 Agonists FDA Approvals & Safety Status
| Drug | FDA Approved Indications (2025) | Black Box Warnings |
| Ozempic | T2DM, CV risk reduction, CKD (Jan 2025) | Thyroid C-cell tumors |
| Wegovy | Obesity, OSA; MASH review Q4 2025 | Thyroid tumors |
| Trulicity | T2DM, CV risk reduction | Thyroid tumors, pancreatitis |
| Mounjaro | T2DM; HFpEF withdrawn | Thyroid tumors, pancreatitis |
| Rybelsus | T2DM; CVOT pending Oct 2025 | Thyroid tumors |
Complete List of GLP-1 Receptor Agonists Available in 2026
The GLP 1 medication list has grown substantially since 2005 offering healthcare providers and patients multiple options across different formulations, dosing schedules and approved indications.
GLP-1 Agonists FDA-Approved for Type 2 Diabetes
Semaglutide (Ozempic)
Approved December 5, 2017, Ozempic represents a once weekly subcutaneous injection that revolutionized diabetes care. It delivers potent glycemic control while producing substantial weight loss. The weekly dosing schedule improves convenience and cardiovascular outcome trials have demonstrated significant reductions in major adverse cardiac events among high risk patients.
Dulaglutide (Trulicity)
Trulicity earned FDA approval on September 18, 2014 and quickly became a popular weekly injection for type 2 diabetes. It features a ready to inject pen that doesn’t require routine dose preparation making administration straightforward. Clinical trials demonstrate robust HbA1c lowering and modest weight reduction.
Liraglutide (Victoza)
As one of the earlier once daily GLP-1 options, Victoza received FDA approval on January 25, 2010 for type 2 diabetes management. This daily subcutaneous injection established liraglutide as a well studied medication with cardiovascular benefits demonstrated in the LEADER trial. The shorter half life requires daily administration but allows for relatively quick dose adjustments when needed.
Exenatide Extended-Release (Bydureon BCise)
Bydureon BCise received FDA approval on October 20, 2017 offering a once weekly exenatide formulation. The extended release microsphere formulation provides sustained exenatide levels throughout the week producing effective glycemic control and modest weight loss.
Exenatide Immediate-Release (Byetta)
Byetta holds the distinction of being the first GLP 1 receptor agonist, receiving FDA approval on April 28, 2005. This twice daily injectable established the entire drug class. While newer alternatives have largely supplanted it, Byetta remains available for patients who respond well to its specific profile.
Lixisenatide (Adlyxin)
Adlyxin received FDA approval on July 27, 2016 as a once daily GLP 1 agonist. Its shorter duration of action produces pronounced effects on postprandial glucose through delayed gastric emptying. The medication is particularly effective when combined with basal insulin.
Rybelsus (Semaglutide)
Rybelsus made history with its FDA approval on September 20, 2019 as the first oral GLP 1 receptor agonist. This once daily tablet uses an absorption enhancer to allow semaglutide to survive the acidic stomach environment and reach systemic circulation.
It requires strict administration rules: take on an empty stomach with minimal water, then wait at least 30 minutes before eating or taking other medications. Despite these restrictions, many patients prefer oral therapy over injections.
GLP-1 Agonists FDA-Approved for Weight Management
Semaglutide (Wegovy)
Wegovy’s approval at 2.4 mg weekly brought unprecedented weight loss efficacy to clinical practice. The STEP trial program demonstrated substantial weight reductions with many participants achieving losses approaching those seen with metabolic surgery. This once weekly subcutaneous injection is specifically indicated for chronic weight management in adults with BMI ≥30 kg/m² or ≥27 kg/m² with at least one weight related condition.
Liraglutide (Saxenda)
Saxenda received FDA approval on December 23, 2014 as the first GLP 1 receptor agonist specifically indicated for chronic weight management. This once daily 3.0 mg subcutaneous injection demonstrated meaningful weight losses in clinical trials with optimal results occurring when combined with reduced calorie diet and increased physical activity.
Dual GIP/GLP-1 Receptor Agonists
Tirzepatide (Mounjaro) – For Type 2 Diabetes
Mounjaro’s May 13, 2022 approval introduced dual GIP/GLP 1 receptor agonism to type 2 diabetes treatment. The SURPASS trial program showed unprecedented glycemic improvements and weight reductions compared with traditional GLP 1 monotherapy. This once weekly subcutaneous injection activates both incretin receptors, producing additive effects that surpass single receptor approaches.
Tirzepatide (Zepbound) – For Weight Management
Zepbound earned FDA approval on November 8, 2023 for chronic weight management. The SURMOUNT clinical program demonstrated substantial weight reductions establishing tirzepatide as one of the most effective obesity medications available. This once weekly injection works through dual GIP/GLP 1 mechanisms to reduce appetite, enhance satiety and improve metabolic parameters.
Discontinued and Phase-Out GLP-1 Medications
Some GLP 1 products have been discontinued or phased out as newer formulations and improved delivery systems emerged. Patients currently using any GLP 1 medication should consult their healthcare provider before making changes as switching between products requires careful dose conversion and monitoring.
How GLP-1 Medications Work in Your Body
When you take a GLP 1 medication, it binds to GLP 1 receptors throughout your body triggering beneficial metabolic effects. The GLP 1 receptor is a G protein coupled receptor that enhances insulin release from pancreatic beta cells in a glucose dependent manner meaning insulin secretion increases primarily when blood sugar is elevated. This targeted response reduces hypoglycemia risk dramatically compared with older diabetes drugs.
Beyond the pancreas, GLP 1 receptors on alpha cells suppress inappropriate glucagon secretion especially during hyperglycemic states. Lower glucagon levels mean your liver produces less glucose contributing significantly to reductions in fasting plasma glucose. These dual pancreatic effects more insulin when needed, less glucagon when harmful, create powerful glycemic control.
The gastrointestinal and appetite effects help to lose weight. GLP 1 medications slow gastric emptying and intestinal transit particularly after meals. This flattens postprandial glucose spikes and help control blood sugar. Meanwhile, GLP 1 receptors in hypothalamic and brainstem nuclei reduce hunger, enhance satiety and shift food preferences away from calorie-dense options.
Recent mechanistic papers also highlight cardiovascular, renal and neuroprotective actions. The LEADER trial demonstrated cardiovascular benefits with liraglutide while other large outcome studies show GLP 1 medications improve endothelial function, reduce blood pressure modestly and favorably modify inflammatory and lipid markers.
The Evolution of GLP-1 Drugs: From Diabetes Treatment to Weight Management
The GLP 1 story began with exenatide’s approval on April 28, 2005 marking the first GLP 1 receptor agonist available for type 2 diabetes. This twice daily injection established the drug class in clinical practice as adjunct therapy for adults not adequately controlled on oral agents.
The landscape shifted with liraglutide. Approved in January 2010 as Victoza for type 2 diabetes, liraglutide introduced once daily dosing and demonstrated cardiovascular benefits. More significantly, its December 23, 2014 approval at higher doses as Saxenda for chronic weight management marked the first GLP 1 agonist specifically indicated for long term obesity treatment.
Semaglutide accelerated this evolution. Its December 5, 2017 approval as once weekly Ozempic for diabetes provided potent glycemic control and significant weight loss in a convenient weekly injection. The September 2019 approval of oral semaglutide (Rybelsus) demonstrated that peptide based GLP 1 therapy could move beyond injections. The culmination came with Wegovy’s June 4, 2021, approval of semaglutide 2.4 mg specifically for chronic weight management.
The newest chapter involves dual agonists. Tirzepatide’s May 13, 2022 approval as Mounjaro for type 2 diabetes introduced the first dual GIP/GLP 1 receptor agonist, producing unprecedented weight loss. Its subsequent November 8, 2023 approval as Zepbound for chronic weight management extended these benefits to people with obesity or overweight plus comorbidity.
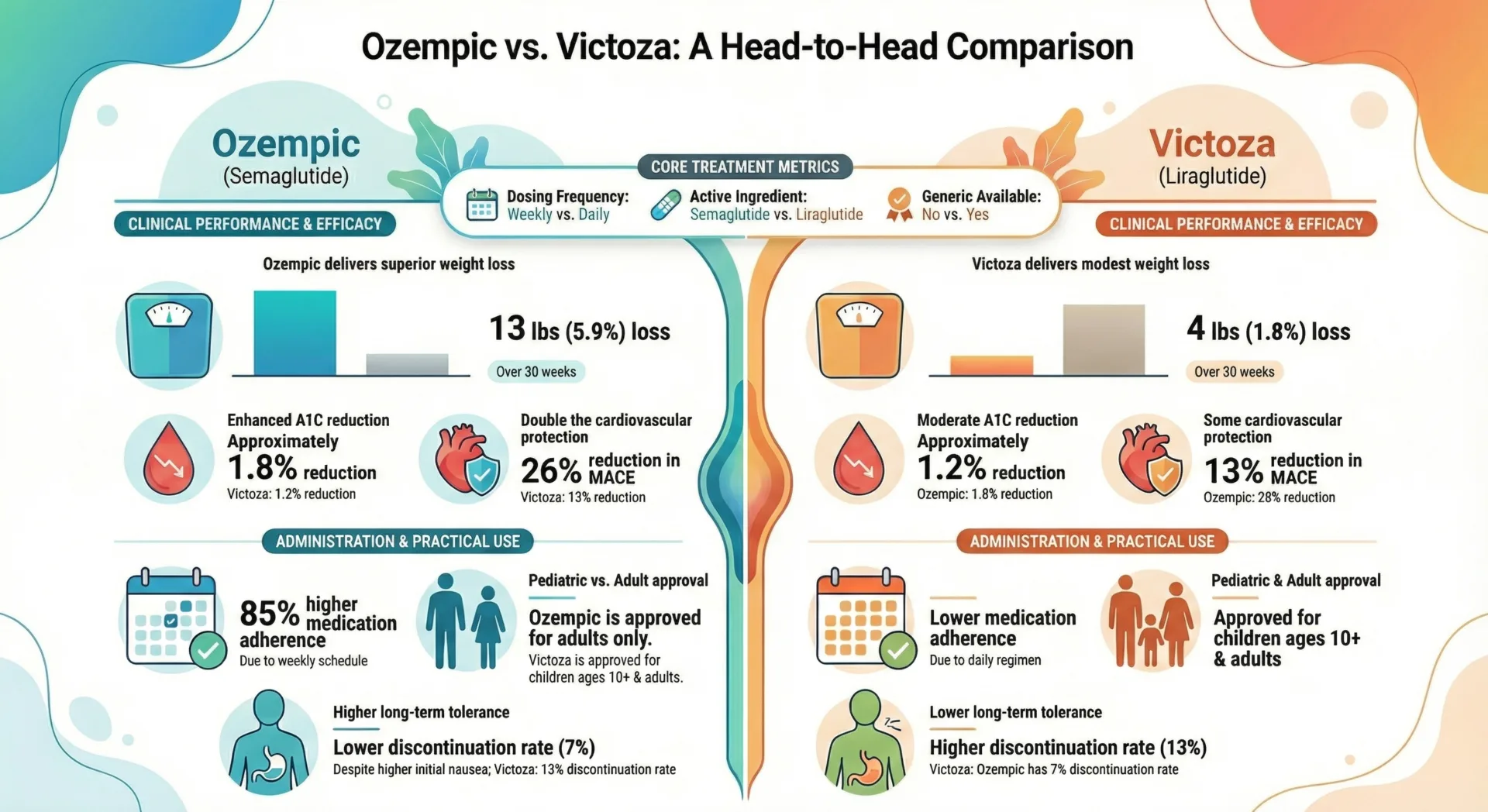
Comparing GLP-1 Medications: Key Differences and Considerations
Choosing among the various GLP 1 options requires understanding key differences in dosing frequency, efficacy and practical considerations around cost and access.
Dosing Frequency: Weekly vs. Daily Injections
Weekly injectables include semaglutide (Ozempic, Wegovy), dulaglutide (Trulicity), tirzepatide (Mounjaro, Zepbound) and exenatide extended release (Bydureon BCise). These formulations use long acting technology to maintain therapeutic drug levels throughout the week. Many patients prefer weekly dosing because it reduces injection burden.
Once daily GLP 1 medications include liraglutide (Victoza, Saxenda) and lixisenatide (Adlyxin), which require subcutaneous injection every day. Some patients appreciate daily dosing because it allows more responsive dose adjustments and produces a predictable routine.
Oral semaglutide (Rybelsus) offers daily tablet administration. While this eliminates injections, it demands strict timing to take on an empty stomach with minimal water, then wait at least 30 minutes before eating or taking other medications.
Efficacy Comparison: Weight Loss and Blood Sugar Control
GLP 1 medications show meaningful differences in their glucose lowering and weight reducing effects. Head to head trials consistently demonstrate that semaglutide formulations produce greater average HbA1c reductions and weight loss compared with earlier GLP 1 options like liraglutide and dulaglutide.
Tirzepatide’s dual GIP/GLP 1 mechanism appears even more potent. Clinical trials comparing tirzepatide against semaglutide showed superior glycemic control and weight reduction with the dual agonist particularly at higher doses. These efficacy differences matter when selecting therapy: patients who need maximal weight loss or have struggled to reach glycemic goals on other agents may benefit most from higher efficacy options.
However, older GLP-1 medications like dulaglutide and liraglutide still produce clinically meaningful outcomes, often with extensive real-world experience and well-characterized safety profiles.
Cost and Insurance Coverage Considerations
GLP-1 medication costs vary substantially, and insurance coverage often determines which options are realistically accessible. List prices for branded GLP-1 medications can range from several hundred to over $1,000 per month without insurance. Most commercial insurance plans cover GLP-1 medications for diabetes, though they may restrict which specific products are preferred or require step therapy.
Coverage for obesity indications (Wegovy, Saxenda, Zepbound) remains more limited. Many insurance plans exclude weight-management medications entirely, leaving patients to pay out-of-pocket. Medicare Part D traditionally excluded weight-loss drugs, though recent policy changes may gradually expand coverage.
Prescription referral services address cost barriers by connecting patients with Canadian pharmacies offering GLP-1 medications at lower prices than typical USA retail. As with any medication purchase, verify pharmacy credentials and discuss with your prescriber before switching sources.
Real-World Patient Experiences and Choosing the Right Medication
What Clinical Guidelines Recommend
Professional societies now position GLP-1 medications prominently in treatment algorithms. For adults with type 2 diabetes and established atherosclerotic cardiovascular disease, high cardiovascular risk, chronic kidney disease, or heart failure, guidelines recommend GLP-1 agonists with proven cardiovascular benefit as preferred agents, independent of metformin use or baseline A1C.
For most adults with newly diagnosed type 2 diabetes without cardiorenal disease, metformin plus lifestyle modification remains initial therapy, with GLP-1 agonists as preferred second-line agents. For people with type 2 diabetes and obesity, guidelines increasingly favor choosing GLP-1-based agents early in the treatment regimen, particularly when weight and cardiorenal risk are central goals.
When BMI and comorbidity thresholds are met for anti obesity pharmacotherapy, GLP-1 agonists are recommended as first line or preferred pharmacologic options because of superior weight loss efficacy and cardiometabolic benefit.
Patient Scenarios: Real World Decision Making
Sarah, 52, with type 2 diabetes and heart disease: Sarah’s provider chose Ozempic over Trulicity specifically because cardiovascular outcome trials demonstrated significant reductions in major adverse cardiac events with semaglutide. Given her history of myocardial infarction two years prior, the proven cardiovascular protection made Ozempic the preferred choice despite both medications offering good glycemic control.
She started at the lowest dose, experienced mild nausea for the first two weeks, and now maintains stable glucose levels with 12% weight loss at 6 months.
Michael, 38, BMI 34, no diabetes: Michael’s physician discussed both Wegovy and Zepbound for weight management. They chose Zepbound because emerging data suggested higher average weight loss with tirzepatide. During titration, Michael experienced significant nausea during weeks 2-4 which his provider managed by slowing dose escalation staying at the starting dose for 6 weeks instead of the standard 4. By month 3, his nausea had largely resolved and he’s down 18 kg at 5 months with good tolerability.
Linda, 65, needle phobic: Linda strongly preferred avoiding injections despite understanding they might be more effective. Her provider started Rybelsus, carefully explaining the strict morning routine: take on truly empty stomach, wait full 30 minutes before coffee or food. Linda found the timing challenging at first but adapted by taking it immediately upon waking, then using those 30 minutes for her morning routine. She’s achieved 8% weight loss and HbA1c reduction from 8.1% to 7.2% over 4 months.
Discontinuation Patterns: What Research Shows
Real world studies reveal that a substantial proportion of patients stop GLP 1 therapy within the first year. Recent data show discontinuation rates reaching significant levels particularly among people using them primarily for weight loss rather than diabetes control. The most common reasons include:
- Gastrointestinal side effects such as nausea, vomiting and diarrhea which are the single most frequently documented reason for stopping in large health system datasets
- High out of pocket cost and lack of insurance coverage particularly in individuals without diabetes
- Medication unavailability or pharmacy supply shortages which peaked in 2023–early 2024
- Perceived completion of therapy once target weight is reached or weight loss plateaus
- Concerns about long term safety or treatment burden of injections
Many cohorts show substantial discontinuation by 3 months, with further losses by 6-9 months. Among those who continue treatment, most report that the worst GI side effects occur during the first weeks of initiation or dose escalation and then improve. Patient reported data suggest that mild to moderate nausea and related symptoms often begin to settle over 2–8 weeks, with further improvement over several months for many but not all patients.
Among those who tolerate and stay on GLP 1 therapy, observational studies show consistent improvements in weight related quality of life, physical functioning, and emotional health over 6–12 months. However, discontinuation is often followed by partial weight regain within months, reinforcing that patients generally experience these medicines as chronic therapies rather than short term cures.
When GLP-1 Medications May Not Be Appropriate
GLP 1 medications are powerful tools but aren’t appropriate for everyone. Consider whether you can commit to permanent lifestyle changes, long term medication use and regular monitoring. Discuss bariatric surgery, behavioral therapy and other evidence based approaches.
These medications may not be suitable if you have severe gastroparesis, active eating disorders, extreme frailty with low BMI, or very limited life expectancy. Weight loss through appetite suppression could worsen sarcopenia in elderly, frail adults. Those with unstable psychiatric conditions or active substance use disorders require careful evaluation before starting appetite modifying medications.
Dosing Guidelines and Administration
Proper dosing and administration techniques maximize GLP 1 medication effectiveness while minimizing side effects.
Standard Dosing Protocols
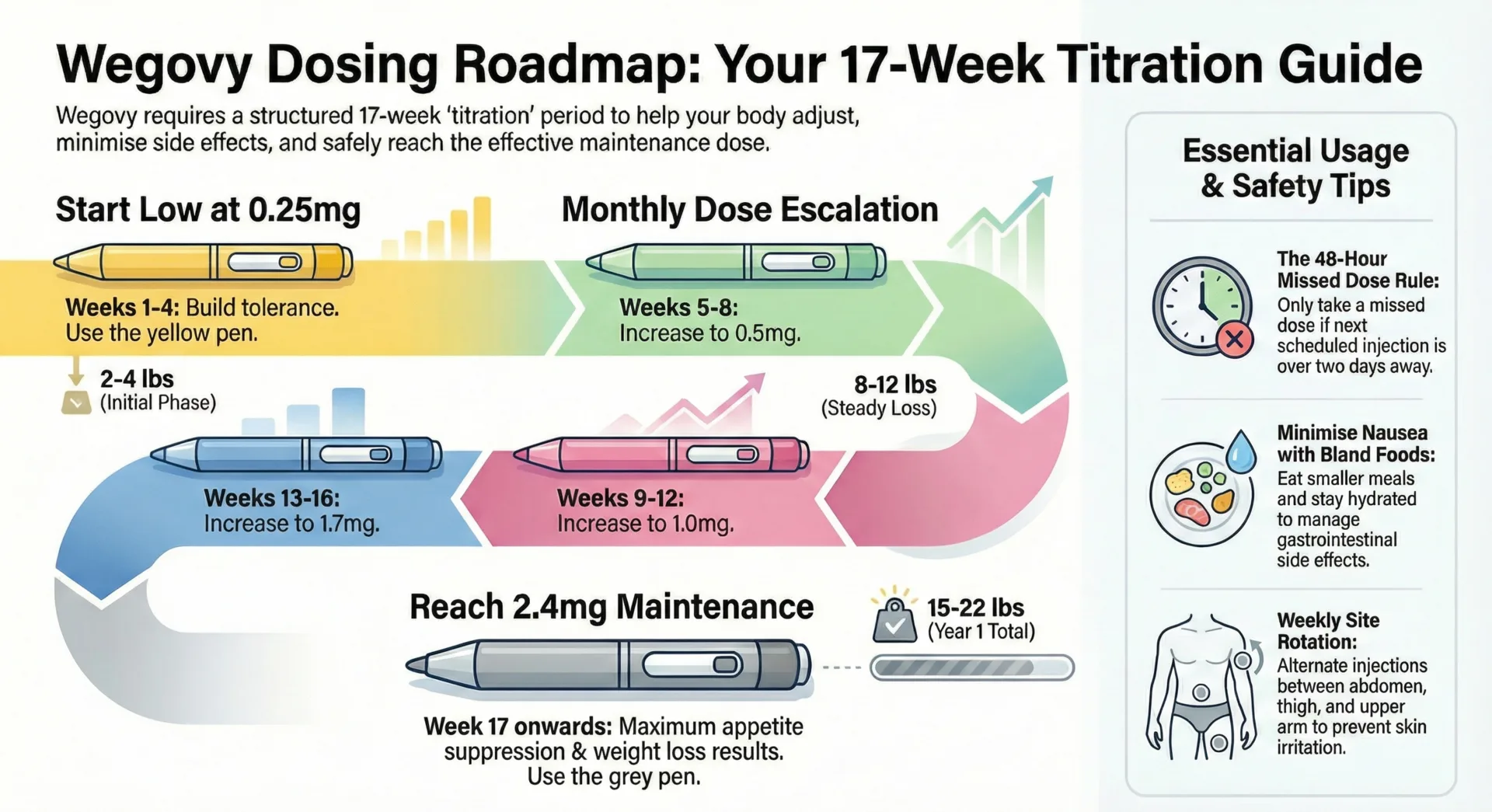
GLP 1 dosing follows a ‘start low, go slow’ philosophy to optimize gastrointestinal tolerability. Weekly injectables like Ozempic begin at low doses with gradual increases every 4 weeks as tolerated. Wegovy follows a similar escalation pattern over 16-20 weeks. Daily injectables like Saxenda start low and increase every week reaching maintenance levels more quickly.
Oral Rybelsus typically starts at 3 mg daily, increases to 7 mg after 30 days and may advance to 14 mg if additional control is needed. These titration schedules balance achieving therapeutic levels with minimizing nausea, vomiting and other gastrointestinal effects that commonly occur when GLP 1 medications are started or increased too quickly. Healthcare providers sometimes modify standard schedules based on individual response.
Injection Techniques and Best Practices
Subcutaneous GLP 1 injections are administered into fatty tissue just below the skin typically in the abdomen, thigh or upper arm.
Before injecting, check the medication for particles or discoloration, let it warm to room temperature if refrigerated and never shake the pen vigorously. Clean the injection site with an alcohol swab and allow it to dry completely.
Pinch up a fold of skin, insert the needle at a 90 degree angle and push the plunger fully to deliver the complete dose. Hold the needle in place for several seconds after injecting, then withdraw smoothly. Rotate injection sites regularly to prevent lipodystrophy and scarring that can affect absorption.
Side Effects and Safety Profile
Common Side Effects and Timeline for Resolution
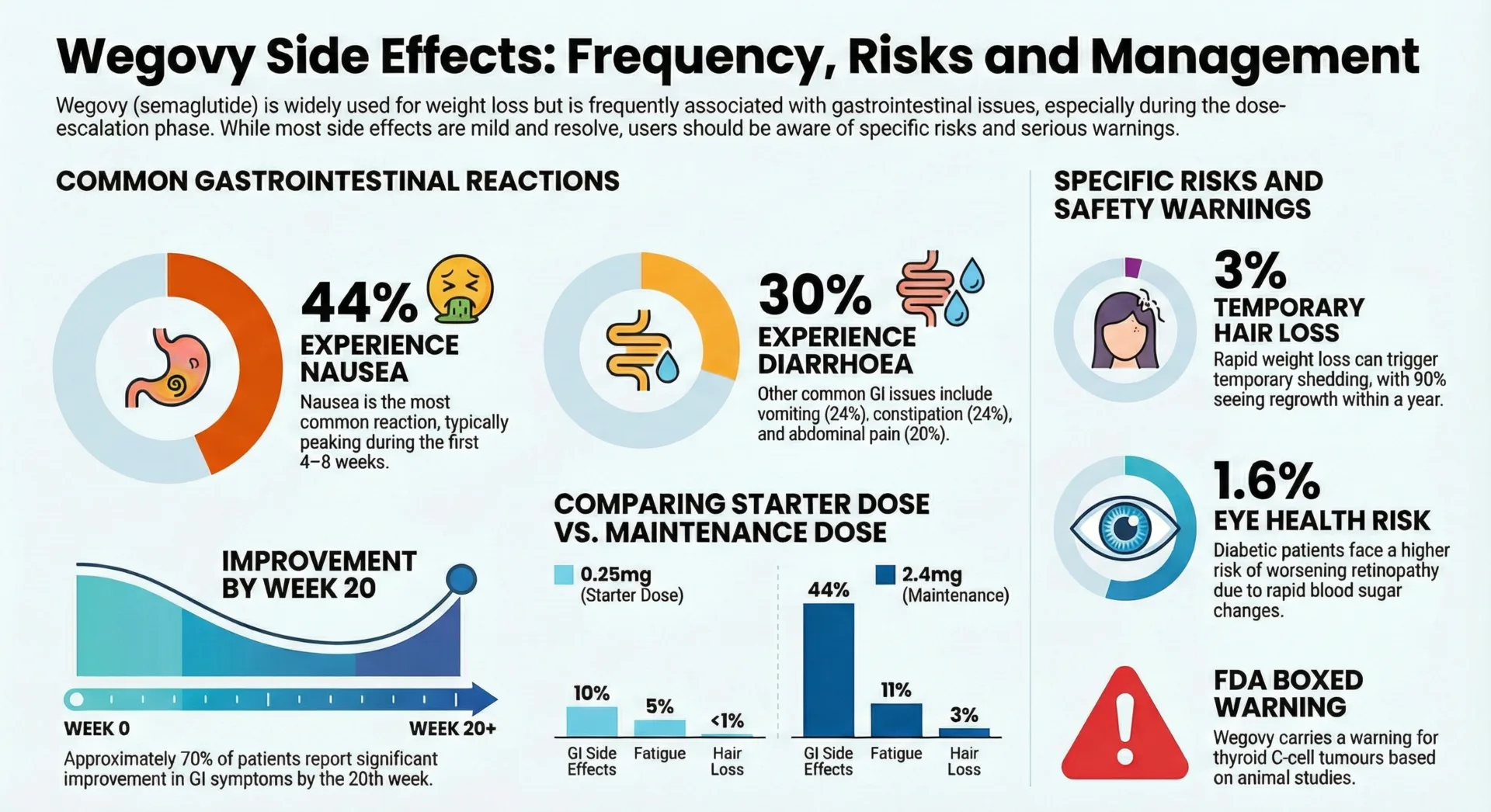
Nausea stands as the most frequent side effect affecting substantial numbers of patients depending on the specific medication and dose. This stems from GLP 1’s effects on gastric emptying and central nausea circuits. Nausea typically peaks during dose escalation and often improves with time at stable doses. Eating smaller meals avoiding high fat or spicy foods and staying well hydrated help many patients manage this symptom.
Vomiting, diarrhea and constipation occur less frequently but still affect notable numbers of patients. Other common gastrointestinal effects include abdominal pain, bloating and early satiety. These symptoms usually diminish over weeks to months as your body adapts.
What to Expect: Timeline for Side Effects
- Week 1-2: Expect mild to moderate nausea which typically peaks 2-3 days after injection. Early satiety and reduced appetite are common. Small, bland meals help most patients manage this period.
- Week 4-6: GI symptoms usually show noticeable improvement for many continuing users. If nausea remains severe at this point, discuss with your provider before advancing doses.
- Week 8-12: Most patients who tolerate the medication find GI effects have stabilized or largely resolved. If nausea persists beyond 8 weeks at the same dose, discuss whether a slower titration or alternative medication would be better.
Injection site reactions: redness, itching or mild pain occur occasionally. Rotating sites and proper technique reduce this risk. Headache, fatigue and dizziness represent additional common but usually mild side effects. Hypoglycemia risk increases primarily when GLP 1 medications are combined with insulin or sulfonylureas.
Serious Adverse Effects and Warning Signs
Though rare, serious adverse effects require immediate medical attention. Acute pancreatitis has been reported with GLP 1 medications typically presenting as severe, persistent abdominal pain that may radiate to the back. If you experience such pain, stop the medication and seek emergency care.
Gallbladder disease including gallstones and cholecystitis, appears more frequently in GLP 1 users. Rapid weight loss may contribute to gallstone formation. Right upper abdominal pain particularly after meals suggests possible gallbladder problems.
All GLP 1 medications with a rodent C-cell tumor signal carry a boxed warning about potential thyroid C-cell tumor risk. These medications are contraindicated in people with personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia type 2.
Acute kidney injury has occurred in patients experiencing severe vomiting, diarrhea, and dehydration. Maintaining adequate hydration and monitoring kidney function during significant gastrointestinal symptoms is essential.
Recent guidance highlights aspiration risk during anesthesia due to delayed gastric emptying. If you’re scheduled for surgery or procedures requiring sedation, inform your anesthesiologist about your GLP 1 medication.
Managing Side Effects: Practical Strategies
Start by eating smaller, more frequent meals rather than three large meals daily. Choose bland, easily digestible foods during dose escalation, avoiding high fat, greasy or spicy options that worsen nausea. Stay well hydrated throughout the day sipping fluids regularly.
Timing matters for oral Rybelsus users. Taking the medication first thing in the morning on a truly empty stomach, then waiting the full 30 minutes before eating, optimizes absorption. For injectable medications, some patients find evening injections reduce daytime nausea.
If symptoms persist or worsen, don’t simply power through. Contact your healthcare provider to discuss dose adjustments, temporary dose reductions or anti nausea medications. Many patients who initially struggle with side effects tolerate the medication well after adjustments to their escalation schedule.
Contraindications and Drug Interactions
Who Should Not Take GLP 1 Receptor Agonists?
Personal or family history of medullary thyroid carcinoma represents an absolute contraindication. Multiple endocrine neoplasia type 2 (types 2A and 2B) also contraindicates GLP 1 use. Previous serious hypersensitivity reaction: anaphylaxis, angioedema or severe allergic responses to a specific GLP 1 medication mean you shouldn’t be re-exposed to that agent.
Pregnancy contraindicates weight loss GLP 1 medications entirely. For diabetes management during pregnancy, insulin and other well studied options are strongly preferred. Women of childbearing potential using GLP 1 medications should maintain reliable contraception.
Active pancreatitis or strong history of GLP 1 associated pancreatitis generally means avoiding these medications. Severe gastroparesis or significant gastrointestinal motility disorders also warrant avoidance as GLP 1 induced gastric emptying delays can worsen existing symptoms.
Very elderly, frail adults with limited life expectancy and high sarcopenia risk may suffer more harm than benefit from aggressive weight loss. Patients with active eating disorders should avoid appetite suppressing medications that could exacerbate disordered eating.
Important Drug Interactions
The most significant interaction involves insulin and sulfonylureas. Combining GLP 1 medications with these glucose lowering agents substantially increases hypoglycemia risk. Labels typically recommend reducing insulin or sulfonylurea doses when starting GLP 1 therapy, with close glucose monitoring.
Delayed gastric emptying affects oral drug absorption particularly for medications requiring timely uptake. Oral contraceptives, antibiotics and narrow therapeutic index drugs may show altered absorption kinetics. Several labels advise considering alternative contraception during dose escalation when gastric emptying effects are most pronounced.
Anesthetic agents interact indirectly through aspiration risk. GLP 1 mediated delayed gastric emptying means residual stomach contents may remain despite standard fasting. Recent guidance recommends individualized perioperative plans potentially holding doses before elective surgery.
2025 Updates: What’s New in the GLP-1 Space
Recently Approved Medications and Indications
Recent regulatory activity has expanded the therapeutic scope of existing GLP 1 medications. FDA approvals now encompass chronic kidney disease indications for some agents, obstructive sleep apnea treatment and metabolic dysfunction associated steatotic liver disease for select products.
Cardiovascular and renal outcome trials continue producing evidence that shapes guideline recommendations. Multiple professional societies now position GLP 1 medications with proven cardiovascular benefits as preferred options for people with type 2 diabetes and established atherosclerotic cardiovascular disease or very high risk.
Compounded Semaglutide and Tirzepatide: Safety Concerns
FDA enforcement discretion policies during drug shortages have allowed compounding pharmacies to prepare semaglutide and tirzepatide products when commercial supplies were insufficient. These compounded versions raise significant safety concerns. Major medical organizations warn against routine use of compounded GLP 1 preparations because they are not FDA approved and can have dosing inaccuracies, formulation variability and impurities linked to serious adverse events.
Compounded products lack the rigorous manufacturing standards, stability testing and quality oversight that FDA approved medications undergo. Reports of incorrect dosing, contamination and unexpected severe reactions have emerged. Patients currently using compounded semaglutide or tirzepatide should discuss transitioning to FDA approved formulations with their healthcare provider.
Supply Chain Status
GLP 1 medication availability, particularly for high demand products like semaglutide and tirzepatide, has experienced significant fluctuations. Manufacturers have substantially expanded production capacity and supply has gradually improved for most products through 2024 into 2025. Prescription referral services can sometimes access Canadian supply channels less affected by USA specific shortage dynamics.
Pipeline Medications
Multiple oral GLP 1 formulations beyond Rybelsus are in late stage clinical trials. Triple agonists combining GLP 1, GIP and glucagon receptor activity represent another frontier with early data suggesting these may produce even greater weight loss and metabolic improvements. Long acting formulations requiring monthly or less frequent dosing could further improve adherence.
Disclaimer: This information is intended for general knowledge and informational purposes only and does not constitute medical advice. Always consult with a healthcare professional for personalized guidance.
Written by the Pandameds.com Editorial Team
Our content is created by pharmacy-trained researchers and healthcare specialists and rigorously reviewed by a diverse panel of authentic experts from the pharmaceutical and healthcare fields. This collaborative review process ensures that every article meets the highest standards of medical accuracy, reliability, and relevance.
- ✅ Authored by pharmacy-trained professionals
- 🔍 Reviewed by multiple verified experts in the pharmaceutical and healthcare niche
- 💊 Based on trusted sources including FDA, Health Canada, and peer-reviewed clinical studies
- 🔄 Regularly reviewed and updated every 90 days to maintain accuracy and trustworthiness
About Pandameds.com
Pandameds.com offers a range of weight loss medications at an affordable price.
Fast, Reliable Shipping to the USA!
Affordable Prescription Meds From Canada
Join our mailing list for exclusive promos, curated health content & more.
Frequently Asked Questions
Who should avoid GLP-1?
Anyone with personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia type 2 should not take GLP 1 medications. Previous serious hypersensitivity reactions, current pregnancy (especially for weight-loss indications), active pancreatitis and severe gastroparesis generally contraindicate use. Patients with eating disorders, extreme frailty or very low BMI may experience more harm than benefit.
Can GLP-1 cause UTI?
Current evidence suggests GLP 1 receptor agonists do not increase urinary tract infection risk. Recent systematic evaluations found no higher rate of UTIs with GLP 1 therapy compared with other diabetes medications.
What is a GLP-1 receptor agonist oral treatment?
The only FDA-approved oral GLP 1 receptor agonist is oral semaglutide, branded as Rybelsus. This once daily tablet uses an absorption enhancer to allow the semaglutide peptide to reach systemic circulation intact. Rybelsus must be taken on an empty stomach with minimal water with at least 30 minutes before consuming food or other medications.
Is Trulicity the same as Rybelsus?
Trulicity and Rybelsus are both GLP 1 receptor agonists for type 2 diabetes but differ significantly. Trulicity contains dulaglutide designed for once weekly subcutaneous injection. Rybelsus contains semaglutide formulated as an oral tablet requiring once daily administration on an empty stomach with strict timing. Head to head comparisons show semaglutide produces greater mean HbA1c reduction and weight loss than dulaglutide at clinically relevant doses.
Related Blog Posts
Call Us Today!
If you have any questions, please email our support team at support@pandameds.com or call us toll-free at 1-888-862-1210.