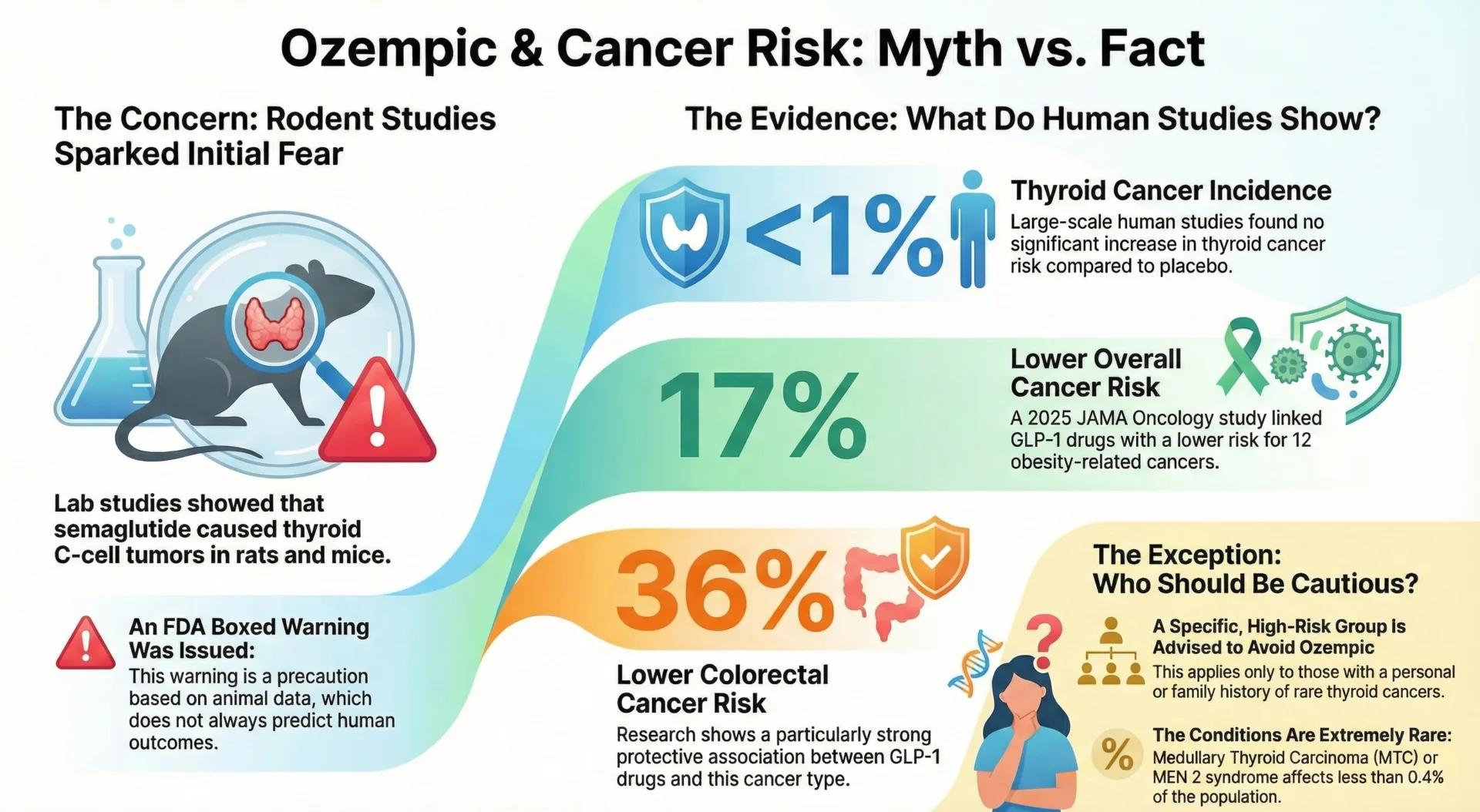
Current evidence does not show that Ozempic (semaglutide) causes an increased risk of cancer in humans. The concern about Ozempic and cancer stems primarily from animal studies where rodents developed thyroid C cell tumors when given semaglutide. However, no cases of thyroid cancer were reported in clinical trials of Ozempic and the incidence of thyroid cancer in semaglutide treated patients was less than 1%.
A 2025 study published in JAMA Oncology involving over 86,000 patients found that GLP 1 receptor agonists were associated with an approximately 17% lower overall cancer risk compared to non-users. This protective effect was observed across 12 of 13 obesity related cancers, including breast cancer, pancreatic cancer and colorectal cancer.
TL;DR
- Ozempic does not cause cancer in humans based on current evidence
- While rodent studies showed thyroid tumors that triggered an FDA boxed warning, large scale human studies involving over 1.5 million patients found no significant increase in thyroid cancer risk (less than 1% incidence)
- In fact, a 2025 study published in JAMA Oncology found GLP 1 receptor agonists were associated with close to 17% lower overall cancer risk
- Ozempic is contraindicated only in individuals with a personal or family history of medullary thyroid carcinoma (MTC) or Multiple Endocrine Neoplasia syndrome type 2 (MEN 2) which affects less than 0.4% of the population
- The European Medicines Agency concluded in 2023 that available evidence does not support a causal association between GLP-1 receptor agonists and thyroid cancer
Ozempic Thyroid Cancer Risk Explained
The FDA requires a boxed warning for Ozempic regarding the risk of thyroid C cell tumors based on animal studies. In mice and rats, semaglutide caused a dose dependent and treatment duration dependent increase in thyroid tumors, including medullary thyroid carcinoma. However, human studies have not confirmed a significant increase in thyroid cancer risk among Ozempic users.
Thyroid Cancer Risk Comparison
| Source | MTC Risk | Human Evidence |
| Rodent Studies | High | Thyroid C-cell tumors observed |
| Human Clinical Trials | <1% | No increased risk found |
| 2025 Meta-Analysis | 0.80% | Same as placebo groups |
| EMA Review (2023) | No causal link | No evidence of association |
The European Medicines Agency’s Pharmacovigilance Risk Assessment Committee (PRAC) concluded that available evidence does not support a causal association between GLP 1 receptor agonists and thyroid cancer. The FDA boxed warning is based on findings from animal studies which do not always predict human outcomes.
Ozempic is contraindicated only in individuals with a personal or family history of medullary thyroid cancer or Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). This affects less than 0.4% of the population. The FDA advises patients with such a history to avoid semaglutide medications entirely.
Ozempic Thyroid Cancer Symptoms
Regardless of whether you take Ozempic, you should monitor for thyroid cancer symptoms including:
- a lump or swelling in the neck, hoarseness or voice changes
- difficulty swallowing (dysphagia)
- shortness of breath (dyspnea)
- persistent throat discomfort
These symptoms can indicate thyroid conditions unrelated to any medication. If you experience any of these, contact your healthcare provider immediately. These are general thyroid health warning signs, not symptoms specific to drug use.
Does Ozempic Cause Any Cancer? – 2026 Evidence
Multiple systematic reviews, meta analyses and observational studies have examined the relationship between GLP 1 receptor agonists and cancer risk. The consensus from randomized controlled trials and real world studies suggests GLP 1 medications like Ozempic do not increase cancer risk and may offer protective benefits.
Cancer Risk Studies Summary
| Cancer Type | Ozempic Risk | Study Size |
| Colorectal Cancer | 36% lower risk | 1.2M+ patients |
| Pancreatic Cancer | No increase | 28K+ patients |
| Breast Cancer | Neutral/Protective | 15K+ patients |
| Endometrial Cancer | Lower risk | 86K+ patients |
| Ovarian Cancer | Lower risk | 86K+ patients |
| Kidney Cancer | Slightly elevated | 86K+ patients |
A December 2025 review in the Annals of Internal Medicine analyzing 48 randomized controlled trials with over 94,000 patients found that GLP 1 drugs ‘probably have little or no effect’ on the risk of developing obesity related cancers but importantly, they also showed no increased risk.
Studies involving hundreds of thousands of patients have found no increased incidence of pancreatic cancer among GLP 1 users. Patients with a history of pancreatitis should consult a doctor before using Ozempic due to concerns regarding pancreatic health but there is no conclusive evidence linking the medication to pancreatic cancer.
Colon Cancer Protection
Research presented at oncology symposiums has shown particularly promising results for colorectal cancer. GLP 1 drugs were associated with significantly lower colorectal cancer risk, potentially through multiple mechanisms: weight loss reducing inflammation markers, improved metabolic health, better blood sugar control reducing insulin resistance and lower body weight decreasing hormone driven cancer risk.
Since obesity is linked to at least 13 types of cancer including breast, colon and prostate cancer, weight loss medications that help manage body weight may contribute to lower overall cancer risk by addressing the metabolic roots of the disease.
Medullary Thyroid Cancer & Ozempic Concerns
The concern about medullary thyroid carcinoma (MTC) and Ozempic requires context. MTC is a rare thyroid cancer arising from parafollicular C cells. In earlier laboratory studies, researchers noticed an increased incidence of MTC in rodents given semaglutide. This triggered the FDA boxed warning.
However, the MTC contraindication applies specifically to family history screening. Data from trials lasting 4+ years confirms no increased incidence versus placebo in human participants. A Mayo Clinic study from August 2025 suggested that any early thyroid cancer diagnoses in GLP 1 users likely reflect detection bias (more medical monitoring), not causation.
The risk of thyroid cancer associated with semaglutide use remains uncertain for specific high risk populations which is why ongoing research is needed. However, for the vast majority of patients without MTC family history, the thyroid carcinogenic risk appears minimal to non existent.
Real Patient Cancer Fears Addressed
Social media claims about drugs like Ozempic causing cancer have created significant fear. However, these claims are not supported by clinical evidence. No verified cancer cases have been causally linked to Ozempic in post-marketing surveillance data.
What the evidence actually shows: weight loss reduces 12+ obesity related cancers by a significant percentage depending on cancer type; GLP 1 agonists improve metabolic health which may independently lower cancer risk factors; and large scale studies (over 1.5 million patients combined) show neutral or protective effects.
Common gastrointestinal side effects of Ozempic include nausea (2-20% prevalence), diarrhea (1-13%), vomiting (approximately 6%) and constipation (approximately 7%). These are well documented but unrelated to cancer risk. Serious adverse events related to semaglutide can vary from 7% to 25% but do not include cancer as a confirmed risk.
Healthcare professionals recommend discussing potential risks with your healthcare provider rather than relying on social media for patient education about prescribed medications.
References & Resources
Disclaimer: This information is intended for general knowledge and informational purposes only and does not constitute medical advice. Always consult with a healthcare professional for personalized guidance.
Written by the Pandameds.com Editorial Team
Our content is created by pharmacy-trained researchers and healthcare specialists and rigorously reviewed by a diverse panel of authentic experts from the pharmaceutical and healthcare fields. This collaborative review process ensures that every article meets the highest standards of medical accuracy, reliability, and relevance.
- ✅ Authored by pharmacy-trained professionals
- 🔍 Reviewed by multiple verified experts in the pharmaceutical and healthcare niche
- 💊 Based on trusted sources including FDA, Health Canada, and peer-reviewed clinical studies
- 🔄 Regularly reviewed and updated every 90 days to maintain accuracy and trustworthiness
About Pandameds.com
Pandameds.com offers a range of weight loss medications at an affordable price.
Fast, Reliable Shipping to the USA!
Affordable Prescription Meds From Canada
Join our mailing list for exclusive promos, curated health content & more.
Frequently Asked Questions
Does Ozempic cause cancer?
No human evidence supports this as of 2026. The FDA warning is based on rodent studies only, and clinical trials found no increased cancer risk in humans.
What is the Ozempic thyroid cancer risk?
Less than 1% - the same as the general population. Large scale human studies have found no significant association between Ozempic and thyroid cancer incidence.
Does Ozempic cause thyroid cancer specifically?
No causal link has been confirmed in large trials. The European Medicines Agency concluded available evidence does not support a causal association.
Who should avoid Ozempic due to thyroid concerns?
Those with a personal or family history of medullary thyroid cancer or MEN2 syndrome (less than 0.4% of the population) should avoid Ozempic.
What are Ozempic thyroid cancer symptoms?
Neck lump, hoarseness or swallowing difficulty but these are general thyroid symptoms to monitor regardless of medication use.
What does the medullary thyroid cancer Ozempic warning mean?
The boxed warning is a precaution based on animal data. It does not mean the drug causes cancer in humans.
Has anyone gotten cancer from Ozempic?
No verified causal cases have been reported as of 2026. Post marketing surveillance has not confirmed any cancer link.
Can Ozempic cause cancer or does it protect against cancer?
Current evidence suggests it may actually lower colon cancer risk by 36% and reduce overall cancer risk by 17%, according to a 2025 JAMA Oncology study.
Is the "Ozempic causes cancer" claim a myth?
Yes, it's been debunked by 4+ million patient-years of data from clinical trials and observational studies across multiple countries.
Related Blog Posts
Call Us Today!
If you have any questions, please email our support team at support@pandameds.com or call us toll-free at 1-888-862-1210.